Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Evaluation of the Cardiac Autonomic Nervous System through Dynamic Exercise and its Relationship to Right Ventricular Diastolic Function in Patients with Type I Diabetes Mellitus
*Corresponding author:Jose Hipolito Donis Hernández, Head of the Merida Cardiovascular Research Institute, Venezuela.
Received:May 05, 2023; Published:May 17, 2023
DOI: 10.34297/AJBSR.2023.18.002523
Abstract
Background: Subclinical right ventricular diastolic dysfunction (RVVDD) is prevalent in the population of patients with type 1 diabetes mellitus (DM1). However, the association between cardiac autonomic neuropathy (CAN) and RVVDD has not been studied. To study in type 1 diabetic patients the association between CAP and the presence of impaired right ventricular diastolic function. Materials and Methods: An observational, analytical, cross-sectional study was carried out in 48 patients, 25 with DM1 and 23 control subjects without DM1. All patients underwent determination of glycosylated hemoglobin (HbA1c), fasting blood glucose, B-mode echocardiogram-Doppler, and Tissue Doppler (TDI) and the Valsalva test as a Cardiac Autonomic Reflex Test (CART). Similarly, the resting heart rate was evaluated and through the performance of a stress test the HR acceleration was determined 10 seconds after the start of exercise and the HR recovery deltas (HRR) at the 1st and 2nd minute after the end of exercise. Results: Right ventricular diastolic dysfunction was present in 13 of the patients with DM1 corresponding to 52% manifested by an altered E/e ratio’ (p<0.039). The Valsalva index ratio was lower in 14 of the diabetic patients (56%) in relation to nondiabetic controls with statistically significant difference (1.31±0.14 vs 1.50±0.082 p<0.0001). CFR was found significantly altered in diabetics compared to controls (delta at 1st min p<0.01, delta at 2nd min p<.0001). Logistic analysis showed that the Valsalva index was the independent variable of highest value with the presence of DDVD since all 13 diabetic patients with DDVD had abnormal Valsalva index. Correlation was obtained between the Valsalva test and the E/e’ ratio with glycosylated Hb and fasting glycemia, with a direct, positive and significant linear relationship of the Valsalva index with glycosylated Hb (r=0.829; p=0.0001) and for its part the E/e’ ratio showed a direct and positive linear relationship with glycosylated Hb (r=409; p=0.042). Conclusion: The alteration of the cardiac autonomic nervous system manifested mainly by an abnormal Valsalva index ratio was associated with DDVD. and in turn this variable was associated with poor metabolic control.
Keywords: Right ventricle, Right ventricular diastolic dysfunction, Diabetic cardiomyopathy, Cardiac autonomic neuropathy
Introduction
Diabetes mellitus is currently one of the most common chronic non-communicable diseases in contemporary societies around the world, one of the five leading causes of death in most developed countries and an increasingly recurrent epidemiological phenomenon in many developing or newly industrialized nations. Among the different types of diabetes, type 1 Diabetes Mellitus (DM 1) is recognized as a condition due to the autoimmune destruction of pancreatic cellsβ resulting in an absolute insulin deficiency [1]. However, this is not the only mechanism and insulin resistance can coexist with what is known as “double diabetes”[2]. DM1 accounts for only about 10% of diabetes cases worldwide, but it occurs with increasing incidence in early life [3]. Diabetes affects the heart in three ways: by coronary artery disease through a mechanism of accelerated atherosclerosis, by cardiac autonomic neuropathy and by the development of Diabetic Cardiomyopathy (DCM). This last mechanism of myocardial involvement not associated with coronary atherosclerosis or systemic Arterial Hypertension (AHT) has also been called Cardiac Muscular Disease (DCMD) [4]. Diabetes mellitus, even in the absence of any other cardiovascular disease, can lead to ventricular dysfunction and clinical CMD, which was first reported by Rubler, et al. [5] in 1972 who found in post-mortem studies of 4 diabetic patients who died of Heart Failure (HF), the presence of ventricular hypertrophy and myocardial fibrosis unrelated to Coronary Artery Disease (CAD), HT, alcoholism, valvular or congenital disease. The prevalence of CMD has been estimated at 12% of people with diabetes mellitus, although the true prevalence is not known due to the subclinical nature of this entity. Initial studies placed it at around 30% in type 2 diabetics with good glycemic control when assessing diastolic function [6-7]. However, when more advanced techniques have been used to evaluate systolic and diastolic function, the prevalence of ventricular dysfunction in the diabetic population is higher than that described in the previously mentioned studies [8-9]. Boyer, et al. [10] have recently reported a higher prevalence of Left Ventricular (LV) diastolic dysfunction when applying tissue Doppler and color M-mode techniques [10]. One of the proposed pathophysiological mechanisms in the development of CMD is cardiac autonomic neuropathy (CAN), i.e., denervation and alterations in catecholamine values [11]. Cardiac Autonomic Neuropathy (CAN) is defined by the Toronto Consensus Panel on Diabetic Neuropathy (2011) [12] as “impaired cardiovascular autonomic control in patients with established diabetes after excluding other causes”. It has been proposed to be associated with alterations in coronary flow and cardiac function, contributing to impaired diastolic function [13-15]. Attenuation of heart rate with respiration, Valsalva maneuver, reduced heart rate variability and reduced heart rate recovery after exercise [16] indicate the presence of NAC. Cardiac Autonomic Reflex Tests (CARTs) are proposed as the gold standard by the Toronto Consensus Panel on Diabetic Neuropathy (2011) to diagnose the presence of CAP. They are 5 simple maneuvers or tests that allow measuring cardiac autonomic function by evaluating the response of Heart Rate (HR) or Blood Pressure (BP) to certain physical maneuvers.
However, heart rate reduction at the 1st and 2nd minutes of post-exercise recovery as a parameter of vagal function together with the measurement of heart rate acceleration at 10 seconds post-exercise have gained importance in recent decades [17]. The prognostic utility of HR recovery at 1 and 2minutes post-exercise has been demonstrated in patients at risk for cardiovascular disease, and has been validated as an independent risk factor compared to other established parameters [18]. CAP has a wide variety of clinical manifestations depending on the stage of the disease, ranging from tachycardia at rest, including alterations in HR variability, HR recovery, QTc interval prolongation, exercise intolerance and even orthostatic hypotension in the advanced stage [19]. In addition to the possible clinical manifestations, the presence of CAP has been associated with left ventricular diastolic dysfunction, as demonstrated in several studies. Several authors have evaluated the type 1 diabetic population in order to assess the existence of early echocardiographic alterations of asymptomatic ventricular dysfunction related to CAP. Irace, et al. [20] in their 1996 publication, already showed that type 1 diabetic patients with CAP had alterations in Left Ventricular (LV) diastolic function expressed as lower E wave velocity and higher transmittal flow A wave velocity, without evidence of alterations in systolic function. Similarly Wille Heimer, et al. [21] found that out of a total of 34 type 1 diabetic patients CAP was present in 21 patients when evaluated with PRAC and the presence of impaired LV diastolic function expressed as a lower E/A ratio was significantly higher in this group with CAP. Monteaguado P, et al. [22] found in their investigation that of a total of 19 type 1 diabetic patients, 11 had CAP and these in turn showed greater presence of LV diastolic dysfunction given by the presence of higher A wave velocity and lower E/A ratio compared to patients without CAP. Hence, they conclude that LV diastolic dysfunction can be detected very early in type 1 diabetic patients and may be related to the presence of CAP. Didangelos, et al. [23] evaluated LV systolic and diastolic function in type 1 diabetic patients with and without CAD and found that 24 patients out of 57 had definite CAD due to the presence of 2 or more altered PRACs and this was related to the presence of an abnormal left ventricular filling pattern by radionuclide ventriculography assessment [23-25]..
They conclude that CAP is associated with LV diastolic dysfunction and suggest that the presence of CAP may serve as an early marker for the assessment of LV diastolic function with a view to implementing therapeutic strategies in a timely manner. [26-28] The finding of a certain degree of diastolic dysfunction in young patients with a recent diagnosis of diabetes or a short duration of the disease, without microangiopathic complications, would suggest that these alterations could appear in the initial stages of the disease and that they would not be related to microvascular involvement, but that other determinants could influence the progression of ventricular dysfunction, such as the presence of CAP [29]. Now, given that right ventricular dysfunction is relevant in a variety of states that affect the course and prognosis of the disease, and therefore, it can be assumed that right ventricular performance is also an important problem in type 1 diabetic patients, most of whom are composed of a young population and who may also have asymptomatic NAC, the aim of this research was to determine the relationship between right ventricular diastolic dysfunction and the presence of alteration of the Cardiac Autonomic Nervous System (CNAS) in patients with type 1 DM who attend the outpatient clinic of the Endocrinology Service of the Hospital Universitario de Los Andes, Merida, Venezuela. Cardiac Autonomic Nervous System (CANS) involvement is related to alterations in right ventricular diastolic function in type 1 diabetic patients [26-29].
Materials and Methods
Research Design
A clinical, observational, analytical, cross-sectional, analytical study was conducted to determine the frequency of right ventricular diastolic dysfunction in patients with type 1 diabetes Mellitus and its association with alteration of the SNAC evaluated through the Valsalva maneuver and stress test in patients who attended the outpatient clinic of the Endocrinology Service of the Hospital Universitario de Los Andes, and subsequently to the Cardiology Service of the Cardiovascular Research Institute, as well as the association with clinical, laboratory and echocardiographic variables, compared to a control group without DM1.
Inclusion Criteria
i. Patients with type 1 DM who attended the Endocrinology Service
consultation, aged between 18 and 45 years of any sex and
race (Study group).
ii. Subjects who attended the consultation of the Endocrinology
Service and/or the Cardiology Service of the Cardiovascular
Research Institute, aged 18 to 45 years, of any sex and race
without a diagnosis of type 1 diabetes mellitus (control group).
iii. Those who agreed to voluntarily participate in the research
and signed the informed consent form.
Exclusion Criteria for the Study and Control Group
i. Patients who did not accept to participate in the research.
ii. Subjects under treatment with hyperglycemic drugs such as:
glucocorticoids, thiazide diuretics, estrogens, progestins, antihypertensives,
B-agonists, pentamidine, interferon alpha,
second generation antipsychotics, antineoplastic medication,
immunosuppressants and antiretrovirals.
iii. Subjects with hematological pathologies such as hemoglobinopathies
or anemia according to the criteria of the World
Health Organization, WHO [25], for those over 15 years of age:
12.4gr/dL at 1500 meters above sea level.
iv. Subjects with systemic HT, hypertensive, ischemic, valvular or
congenital heart disease, cardiac arrhythmias, impaired left
ventricular function (LVEF)<50%, lung disease, chronic kidney
disease grade 3 or higher [22], hypothyroidism, alcoholism.
v. Pregnant patients.
vi. Patients with any physical limitation that would prevent them
from performing a stress test.
Procedure
The patients were randomly selected by the research physician and those who met the inclusion criteria were explained the objective and importance of the study and were asked to voluntarily participate in it and were subsequently given informed consent to sign. Data were collected regarding medical history number, family and personal history, symptomatology, psychobiological habits, age, sex, weight, height, body mass index, blood pressure, fasting and postprandial blood glucose in the last 3 months, glycosylated hemoglobin A1c (HbA1c) and treatments received. Echocardiography was performed by a physician trained in M-mode, two- dimensional and Doppler, with an ALOKA Prosound α7 echocardiography equipment model, with a 3.5MHz Transducer. The measurements were obtained according to the recommendations of the American College of Cardiology [27-29]; with the transducer in the different views, started in the apical view four chambers, at the end of diastole, was obtained through two methods the size of the Right Atrium (RA), with 2D volume measurement and linear measurements, the two measurements obtained were: on the minor axis of the RA (Width of the distance from the lateral wall to the intra-atrial septum, middle atrial level, from the inner edge to the inner edge) and on the major axis of the RA (Length from the center of the tricuspid valve annulus to the center of the upper wall of the RA, parallel to the intra-atrial septum, from the inner edge to the inner edge), and dimensions of the RA were designated in altered ranges, indicative of RA dilatation, minor diameter >44mm and major diameter <53mm. Subsequently, we proceeded to quantify the RA volume (areas of the tricuspid valve leaflets, inferior vena cava, superior vena cava and RA appendage were excluded), an area of right atrial length was traced from the level of the tricuspid valve annulus, which connected the two annular sides with a straight line, the length is perpendicular to the annular plane), which was considered as abnormal a volume of the RA according to gender and body surface area, for men >32ml/m2 and for women >27ml/m2; In the same way, measurements were made of the Right Ventricle (RV), in the different basal, medial and longitudinal diameters, and diameters outside normal ranges were assigned, indicative of RV dilatation, ≥42mm, ≥36mm and ≥84mm respectively, in addition, the thickness of the lateral wall of the RV was measured linearly in two-dimensional echocardiography, in a subcostal view, and at end diastole (below the tricuspid annulus at a distance approximating the length of the anterior tricuspid leaflet, when fully open and parallel to the RV free wall, (trabeculae, papillary muscles and epicardial fat were excluded), a hypertrophic RV was identified with a thickness >0.5cm.
Consecutively, both global and regional RV systolic function was assessed, within the global parameters, Fractional Area Change (FAC) and Myocardial Performance Index (MRI) were measured, both methods are recommended for quantitative estimation of RV function(27-289) , CAF was estimated from a four-chamber apical plane, centered on the RV, to include the apex, being necessary to perform a manual tracing of the endocardial border in diastole and systole and thus calculate the percentage of change, trabeculae were excluded when tracing the area. MRI measures the ratio between isovolumetric contraction and relaxation times in relation to systolic ejection; this quantification was carried out from the Doppler recording, and the Isovolumetric Contraction Time (ICT) was measured, Isovolumetric Relaxation Time (IRT) and Ejection Time (ET) were measured in the pulsed tissue Doppler spectrum of the lateral tricuspid annulus, depressed global RV systolic function was determined if the CAF<35% and if the tissue Doppler MRI is >0.55; in assessing regional RV systolic function, Tricuspid Annulus Systolic Excursion (TAPSE) and S-wave’ parameters were established, TAPSE is a measure of longitudinal RV function, was measured using M-mode and the maximum distance of systolic displacement of the annulus was quantified and for the calculation of the S-wave’ pulsed tissue Doppler was used to quantify the systolic velocity of the myocardial muscle(27-30), as depressed regional systolic function a TAPSE <1.6cm and S-wave’ <10cm/s. We immediately proceeded to analyze the tricuspid flow pattern by measuring: E-wave velocity, A-wave velocity, E/A ratio and E-wave deceleration time, with pulsed Doppler, in addition to e-wave velocity’ using tissue Doppler, in conjunction with the evaluation of the flow in the hepatic veins. The presence and degree of diastolic dysfunction was determined based on the following criteria [27]. I. Grade I: Altered relaxation. Tricuspid E/A Ratio < a 0.8 II. Grade II: Pseudo normal pattern. E/A ratio of 0.8 to 2.1 with an E/e’ ratio> to 6 or predominant diastolic flow in hepatic veins. III. Grade III: Restrictive filling. E/A ratio > to 2.1 with a TDEc < to 120msg
Finally, we proceeded to estimate RV Systolic Pressure (RVSP) and Right Atrial Pressure (RAP). To this end, we determined the velocity of Tricuspid Insufficiency (TI) with continuous Doppler at the level of the aliasing formed in the tricuspid valve and calculated the gradient of the insufficiency using Bernoulli’s equation, as follows: RVSP: 4 x Maximal velocity2 + DBP; said pressure was catalogued elevated if it was > 25mmHg. The DBP was determined according to the size of the Inferior Vena Cava (IVC) and the percentage of collapse of the same, normal right atrial pressures oscillate around 3mmHg if the IVC is <2.1cm and collapsed >50%, increased RA pressures are estimated at 8mmHg, if IVC <2.1cm with collapse <50% or dilated IVC >2.1cm with normal collapse >50%, high DBP at 15mmHg if IVC is dilated >2.1cm with abnormal collapse <50%. Prior to the stress test, the Valsalva maneuver was performed: with the patient in a seated position, the patient was instructed to perform a forced exhalation, without having previously taken a deep inspiration, for 15seconds, followed by an abrupt release of the expiratory effort, and then normal breathing without movement or speaking until the end of the test [30]. Simultaneously, an electrocardiographic trace was recorded in DII to calculate the Valsalva ratio, which was obtained by dividing the longest R-R interval after expiratory effort by the shortest R-R interval during expiratory stress. The normal reference values for the Valsalva maneuver were those published by Ziegler, et al. [31] adapted by age group and gender (Table 1).
The stress test was then performed on GE equipment with electrocardiographic recording (12 leads) computerized in digital format and monitored in real time, connected to a dynamic exercise band with standard Bruce protocol with a cooling period of 2minutes. Vagal reserve was determined as the HR acceleration obtained with the difference between the HR reached 10seconds after the start of exercise minus the basal HR obtained at rest. Post-exercise HR recovery was determined as the difference between the heart rate at maximum effort (maximum heart rate) and the HR at recovery, at 60seconds (Δ1) and 120 seconds (Δ2) after the end of exercise. A normal value of post-exercise heart rate recovery was considered to be a Δ1 greater than 12 beats and a Δ2 greater than 22 beats [32].
Statistical Analysis
Categorical variables were analyzed in absolute numbers and percentages and quantitative variables in mean and standard deviation. The Kolmogorov-Smirnov test was performed to determine whether the variables had a normal distribution. The association between categorical variables was determined by applying Chisquare or Fisher’s test. The differences between the means of the quantitative variables between the groups studied were determined with Student’s t test for independent samples or its nonparametric equivalent. The association between SNAC alterations with the presence of diabetes and the association between SNAC with the presence of right ventricular diastolic dysfunction was performed through association analysis for categorical variables to obtain the Odds Ratio (OR), with chi-square test. In addition, linear correlation analysis was performed between the different biochemical variables and the echocardiographic variables to find out if there were significant correlations and simple linear correlation analysis. Once found in the correlation analysis the variables that were related to the dependent variable, we searched through linear or multiple logistic regression analysis for the independent variables altered in the right ventricle that had greater statistical weight on the dependent variable that was found in the analysis for the two groups studied. An analysis of ROC curves was also considered to find, according to the independent variable altered right ventricular function and altered autonomic function, a cut-off value that could be considered to differentiate the groups studied. A p<0.05 was considered statistically significant.
Results
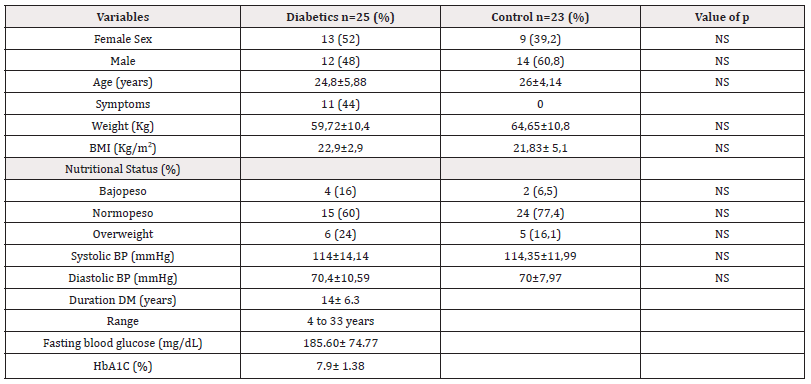
From January to September 2020, a total of 25patients with type 1 Diabetes Mellitus as the study group and 23 healthy persons as the control group, matched for age, sex and body mass index were included in the study. The mean duration of the disease in the diabetic group was 14±6 years. Most diabetic patients showed poor metabolic control with mean HBA1c of 7.9±1.3% in addition to fasting glycemia at 185.6±74.77mg/dl (Table 2).
*Note: Data in X±SD and n (%). NS: not significant; BP: blood pressure. BMI: body mass index.
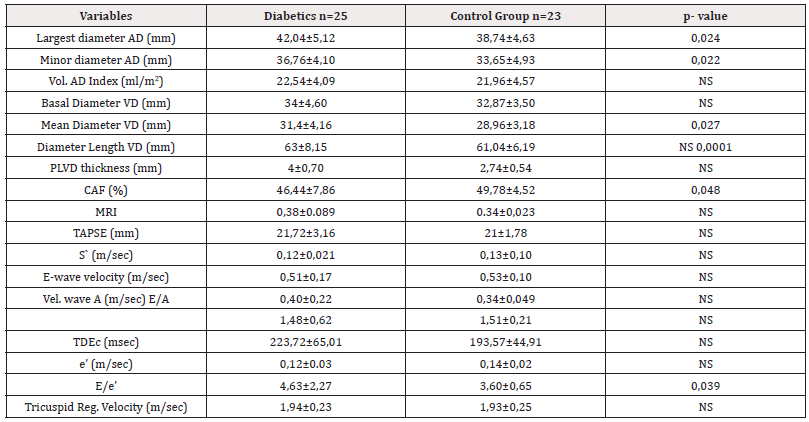
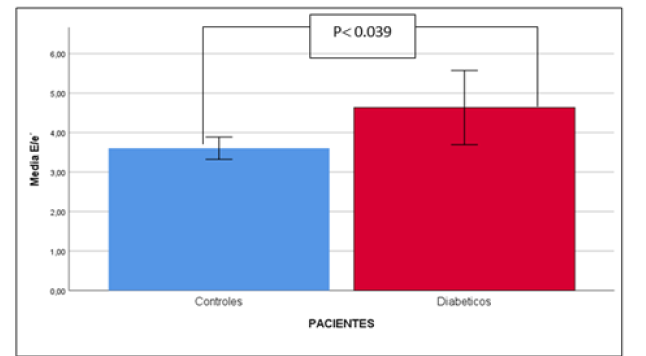
The B-mode echocardiographic variables that showed significant differences in the diabetic group when compared to the control group are shown in Table 3. The only systolic function alteration variable that showed significant difference was the Myocardial Performance Index (MRI) (0.38±0.089 vs. 0.34±0.023; p=0.048). When the criteria for altered Right Ventricular Diastolic Function were evaluated, a significantly increased E/e ‘ratio was found in the diabetic group with respect to the control group (4.63±2.27 vs. 3.60±0.65; p=0.039). (Table 3, Figure 1).
*Note: Data in X±DE and n (%). NS: Not Significant; CAF: Fractional Area Change; MRI: Myocardial Performance Index; cDEV: Deceleration Time; IVC: Inferior Vena Cava; RVLW: Right Ventricular Lateral Wall.
With respect to the variables that assess cardiac autonomic function, the Valsalva ratio was lower in 14 diabetic patients (56%) compared to non-diabetic controls with a statistically significant difference (1.31±0.14 vs. 1.50±0.082 p<0.0001), the basal heart rate at rest in diabetic patients was significantly higher compared to controls (101.84±15.42 vs. 76.78±8.9 p<0.0001). When evaluating vagal reserve measured through HR acceleration 10’’ after starting dynamic exercise with the stress test, it was found to be significantly higher in diabetics compared to controls (111.16±15.97 vs 88.39±21.30 p<0.0001). The other variables that evaluate the recovery of the heart rate through the delta (Δ) at the first and second minute, in diabetics a lower response to the change of response at the first minute was statistically significant (23.92±8.57 vs35.39±12.08 p<0.01) Similarly, the delta (Δ) at two minutes had a similar behavior to the delta (Δ) of the first minute, but with a greater reduction in heart rate response (37.16±11.15 vs 51.39±12.87 p<0.0001) (Table 4).
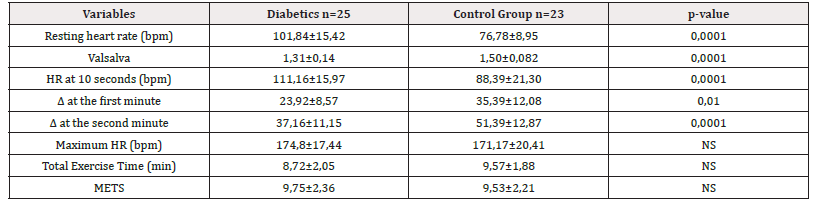
Table 4:Variables of Cardiac Autonomic Function in diabetic patients and control group: Valsalva Test and the variables obtained in the Exercise Test.
*Note: Data in X±DE. NS: Not Significant; HR: Heart Rate; METS: Metabolic Equivalent.
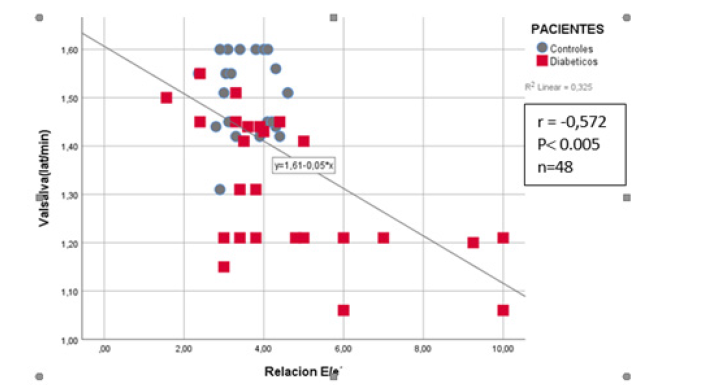
The simple linear correlation analysis between the B-mode echocardiographic parameters with the E/e ratio’ and the Valsalva Index and the cardiac autonomic function variables obtained by stress test showed that the most significant relationship was obtained by the E/e’ ratio, which showed a direct and inverse linear and statistically significant relationship with the Valsalva test (R of -0.572 p<0.005) (Figure 2).
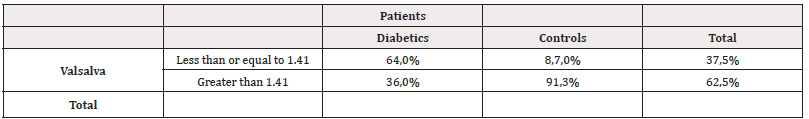
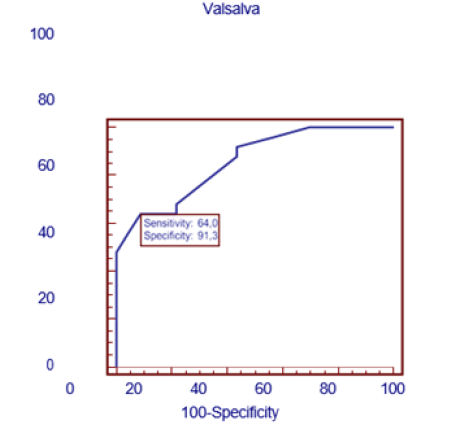
Similarly, a simple linear correlation analysis was performed between resting HR and the presence of DDVI and a positive linear relationship was found between these two variables (R-0.436; p=0.003).With these simple linear correlation results, we proceeded to perform a multiple linear regression analysis , having as dependent variable the E/e ratio’ and as independent variables the variables obtained by M Mode Echo and Trans tricuspid Doppler and found as independent variables the A wave velocity, with a p<0.001, coefficient β= 11.29.(95% CI=9.35.89 to-13.229), the Myocardial Performance Index (MRI) with a p<0.008, coefficient β= 5.08.; (95% CI between 1.38 to 8.79) , the E/A ratio with a p<0.0001, coefficient β= 2.03.(95% CI between 1.37 to 2.69) and the systolic excursion of the lateral tricuspid annulus: TAPSE (mm) with a p<0.04 coefficient β= -0.101; (95% CI between-0.2 to-0.002) . When multiple regression analysis was performed with the independent variables of cardiac autonomic function that had shown significance in the simple correlation analysis, in order to know which remained as the main independent variable with the dependent variable (E/e’ ratio), it was found that the only independent variable was the Valsalva ratio, reaching a level of statistical significance with an R2 of 0.325, p<0.001 with a coefficient β= -6.635. (95% CI between -9.470 to -3.799). As the Valsalva test index was the most significant independent variable in the multiple regression analysis, the cut-off point value of this test between the diabetic group and the control group was sought. For this, a ROC curve was used, finding a value of ≤1.41, to consider an abnormal Valsalva index test with an area under the ROC curve of 0.85; p<0.0001, with Sensitivity values of 64%, and Specificity of 91.3 % (Figure 3). When a 2x2 Table was performed with this value of the Valsalva test with the diabetic patients and the controls, it was found that 16 (64%) of the 25diabetic patients had an alteration of this test, showing statistical significance with a p<0.0006 with respect to the control group, where only 2 (8.7%) individuals showed an altered Valsalva test (Table 5).
*Note: OR: 18.67(CI AL 95 %=3.5 A 98.62) p<0.0006
Right Ventricular Diastolic Dysfunction
When we analyzed the group of diabetics with respect to the presence or not of Right Ventricular Diastolic Dysfunction (RVDDD) we found that 13 patients (52%) of the sample presented RV Diastolic Dysfunction which was catalogued according to the degrees of Diastolic Dysfunction as shown in Table 6. It should be noted that there was no significant relationship between RV Diastolic Dysfunction with respect to the duration in years of DM (Table 6).
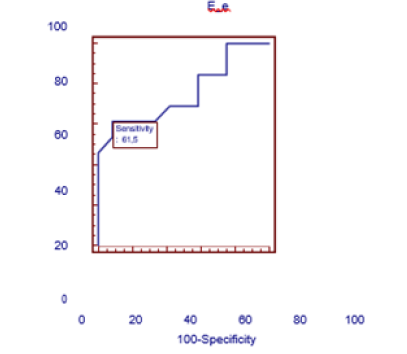
In view of the fact that the E/e’ ratio has implications in right ventricular diastolic dysfunction, a cutoff point for this ratio was also sought to demonstrate the presence of right ventricular diastolic dysfunction exclusively in the group of diabetic patients as a sign of myocardial stiffness. A ROC curve was performed, and the cut-off point obtained was an E/e’ ratio≥4.4 with a sensitivity of 61.5% and a specificity of 91.7% with an OR=17.6 (95% CI 1.7 to181.2) p< of 0.01 (Table 7 and Figure 4). Thus, when we took this cut-off value for the E/e’ ratio we found that 8 (61.5%) of the13 type 1 diabetic patients had this ratio altered and 11 patients (91.7%) of the 12 diabetic patients without DDVD this ratio was at values below 4.4 Table 7.
Similarly, we proceeded to evaluate how diabetics behaved exclusively with respect to this abnormal Valsalva index with the presence or absence of Diastolic Dysfunction of the RV (DDVD) to know to what extent this abnormal Valsalva test could explain this alteration in patients with DDVD with the same cut-off point obtained previously≤1.41 Table 5. We found that all 13 patients (100%) of the total of those with DDVD showed this abnormal index, and only 3 (25%) in the group without DDVD (Table 8).
Finally, a correlation analysis was performed between the Valsalva test and the E/e’ ratio with the variables that measure metabolic control, and a direct, positive and statistically significant linear relationship was found between the Valsalva test with glycosylated Hb (r=-0.829; p=0.0001) and with fasting glycemia values (r=-0.631; p=0.001). In turn, the E/e’ ratio showed a direct and positive linear relationship with glycosylated Hb (r=409; p=0.042). This suggests that the higher the glycosylated Hb value and the higher the fasting glycemia values, the greater the alteration of the Valsalva test and the greater the presence of DDVD.
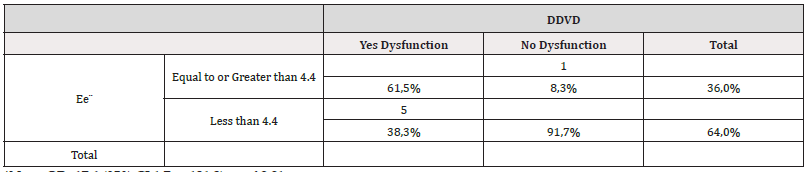
Table 7:Table showing the association of the E/e ratio’ with respect to the presence or absence of RV Diastolic Dysfunction in patients with Diabetes Mellitus.
*Note: OR=17.6 (95% CI 1.7 to 181.2) p< of 0.01
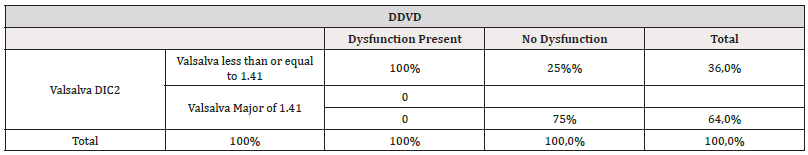
Table 8:Relationship between the Valsalva Index and the presence of right ventricular dysfunction exclusively in diabetics with and without ventricular dysfunction.
*Note: OR=73.2 (95% CI 3.3 to 1590.29) p: 0.006
Discussion
In this clinical, observational, non-randomized research on the evaluation of the cardiac autonomic nervous system and its association with alteration of right ventricular diastolic function in patients with type 1 diabetes mellitus, we first found that the resting HR in diabetic patients was significantly higher than in the controls. Fifty-six percent of diabetics exhibited an altered Valsalva ratio and the variables obtained in the stress test: HR acceleration at 10’’ from the start of dynamic exercise and heart rate recovery through deltas (Δ) at the first and second minute of recovery were also found to be significantly altered in diabetics [33]. Of the 5 Gold Standard Cardiac Autonomic Reflex (CAR) tests proposed by the Toronto Diabetic Neuropathy Consensus Panel (2011 for the diagnosis of cardiac autonomic neuropathy (CAR) we selected the Valsalva maneuver given that a previous investigation carried out in our center by Dr. Gonzalez H, et al. showed that of the5 tests: HR response to deep breathing, HR response to standing, HR response to Valsalva maneuver, blood pressure (BP) response to standing and BP response to a grip test, the Valsalva Index ratio was superior (p<0.0001) as a diagnostic test showing greater sensitivity, specificity and greater negative predictive value. [25] Similarly, the Consensus Panel establishes that at least two of the 5 cardiac autonomic reflex tests must be altered in order to establish a definitive diagnosis of cardiac autonomic neuropathy (CAN). The presence of only one of them altered makes the diagnosis probable. In this sense, given that 56% of our diabetic population presented alteration of the Valsalva maneuver, we can say that these patients have probable CAP. Now, the stress test provides an objective global evaluation of the cardiovascular autonomic nervous system through parasympathetic activation in the post-exercise maximal recovery stage by means of HR recovery [34-36]. Several studies show that diabetic patients present impaired post-exercise HR recovery (RFC) as a reflection of dysautonomia [6-41].Therefore, the finding of significant alteration of HR acceleration 10’’ after the start of exercise and of post-exercise HR recovery deltas in our diabetic population allows us to establish the existence of an attenuated parasympathetic response, but these variables did not correlate with the E/e’ ratio as an indicator of the presence of altered RV diastolic function. Although it is true that these variables obtained in the stress test do not form part of the criteria established by consensus for the diagnosis of CAP, they could be included in the future as autonomic function tests to evaluate these patients. It is known that CAP is a microvascular complication of diabetes, characterized by abnormal regulation of the cardiovascular autonomic nervous system. Its prevalence is underestimated due to the lack of knowledge about it and little active search for signs and symptoms. Diagnosis is relevant since it leads to higher mortality and deterioration of quality of life in diabetic patients. It should be taken into account that the onset of symptoms, which are non-specific, is usually late, when management options are limited. In recent years, publications on the subject have increased, which has allowed a better understanding of its pathophysiology, epidemiology and clinical manifestations. Similarly, CAD is one of the pathophysiological mechanisms involved in the development of Diabetic Cardiomyopathy (DCM). Several studies have confirmed that Cardiac Autonomic Neuropathy (CAD) has an important relationship with the development of LV systolic function impairment expressed as a reduction in ejection fraction (LVEF) and as an alteration of left ventricular filling, which translates into alteration of diastolic function. Similarly, diastolic function abnormalities are more prevalent in diabetic patients with CAP compared to diabetic patients without this complication [42].
CAP is characterized by an alteration in the parasympathetic- sympathetic nervous system balance, in which a reduction in parasympathetic tone secondary to autonomic neuropathy leads to a relative hyperactivity of the sympathetic system. One of the pathophysiological aspects related to cardiac autonomic neuropathy is the development of ventricular dysfunction. This association has been demonstrated mainly for the LV [20-23] and so far nothing has been published in the right ventricle. Therefore, the most important contribution of our research lies in the fact that we demonstrated that 61.5% of diabetic patients with impaired right ventricular diastolic function showed an altered Valsalva index ratio compared to the group without right ventricular dysfunction, 91.7% of whom did not present this alteration, which allowed us to demonstrate that dysfunction of the cardiac autonomic nervous system manifested mainly by an abnormal Valsalva index ratio is associated with DDVD. In relation to the presence of altered RV diastolic function, in our study 52% of patients with type 1 DM presented right ventricular diastolic dysfunction, even with different degrees of dysfunction, with 3 patients showing grade III diastolic dysfunction of the restrictive type. The variable that obtained the greatest significance for the diagnosis of RVVD was the E/e ratio’ which translates into greater myocardial stiffness of the chamber and therefore limits ventricular filling. Similarly, small but statistically significant differences were obtained in the largest and smallest diameter of the RA, mean diameter of the RV and in the thickness of the lateral wall of the RV in diabetics compared to healthy controls; however, these did not reach the values considered abnormal according to the guidelines for Echocardiographic Evaluation of the Right Heart in Adults of the American Society of Echocardiography [27]. The presence of DDVD in diabetic patients has been demonstrated in previous studies [43-47]. Even a study conducted by Khattab, et al. [47] which included type 1 diabetic children and adolescents showed the presence of RV Diastolic Dysfunction given by the presence of decreased tricuspid lateral annulus e’ wave velocity and increased E/e’ ratio which coincides with our findings.
Regarding the presence of RV systolic dysfunction in type 1 diabetics, data are scarce. In our investigation only 2 (8%) of the patients in the diabetic group showed RV systolic dysfunction given by an altered myocardial performance index measured by tissue Doppler. Studies using more advanced echocardiographic techniques such as strain/strain rate have shown a higher prevalence of subclinical systolic dysfunction of both ventricles [46,48]. Diabetic Cardiomyopathy (DCM) is considered a relatively common entity with an estimated prevalence of around 60%, although the true prevalence is not known due to the subclinical nature of this entity. Although RV function has been evaluated in several diseases, most studies analyzing diabetic cardiomyopathy have focused only on the left ventricle. Many of the proposed mechanisms for the impairment of LV function are systemically mediated and, therefore, are also likely to affect RV function and the pulmonary vasculature equally. Despite the important contribution that the RV confers to overall cardiac function, RV dysfunction in CMD has been underestimated. This contribution has been recognized in a variety of disease states, such as Pulmonary Arterial Hypertension (PAH), Coronary Artery Disease (CAD), and arterial hypertension. Several studies have pointed out that RV function plays an important role in risk stratification of patients with heart failure and is considered a strong predictor of atrial fibrillation onset [49,50].
A limitation of our research is the relatively small sample size, which is, however, sufficient to obtain meaningful results. It is worth noting that the total estimated sample could not be reached due to the reasons imposed by the COVID-19 pandemic. In addition, it was a cross- sectional study, Finally, diabetes is associated with profound changes in cardiac metabolism, structure and function. The high prevalence of diastolic dysfunction is due to myocardial fibrosis, and in the Strong Heart Study [42,51] the extent and frequency of diastolic dysfunction was directly proportional to the HbA1c level probably due to the accumulation of advanced glycation end products (AGEs)in the myocardium [52]. Recently, the process of advanced glycation has been directly related to alterations in myocardial calcium handling. It has been shown that advanced glycation of SERCA2a (sarcoplasmic/endoplasmic reticulum Ca2 + -ATPase 2a) leads to a activity and a prolongation of cardiac relaxation [53]. In addition, both myocardial apoptosis and fibrosis have been identified in diabetic subjects, reflecting hormonal changes, particularly affecting angiotensin and aldosterone [54]. The perspective that all these structural and functional changes affect only the left ventricle while the right ventricle remains intact is unreasonable. The two ventricles are anatomically linked by their common blood supply, muscle fiber anatomy, interventricular septum and pericardium [55] and exhibit interdependence as already demonstrated in other conditions such as arterial hypertension. It is also known that poor metabolic control is associated with the appearance of CAP. In this sense, in our investigation we found that most diabetic patients exhibited poor metabolic control given by an elevated HbA1c value and this correlated significantly with the presence of altered Valsalva ratio (p=0.0001). Similarly, elevated fasting glycemia values correlated with the probable presence of CAP (p=0.001). The same significant correlation was found between HbA1c and the presence of DDVD (p<0.042) which also limits the conclusions and the lack of follow-up of the study population, to evaluate the behavior of diastolic function and the evolution of the SNAC alteration and thus be able to know the long-term morbidity and even mortality of this population. Another limitation is the absence of echocardiographic modalities such as strain/strain rate and 3D echocardiography in the center where the study was carried out, which would allow a better evaluation of possible subclinical RV systolic dysfunction and would provide a better assessment of the RV given its complex anatomy.
Conclusions
Diabetes Mellitus is a chronic disease with a very high morbidity and mortality rate, mainly related to cardiovascular disease. Cardiac Autonomic Neuropathy (CAN) is a frequent and underdiagnosed complication of diabetes and is associated with increased mortality from cardiovascular causes. CAP should be recognized as a prevalent and important complication by physicians faced with the care of patients with diabetes mellitus. [25] Our research showed that right ventricular diastolic function is impaired in type 1 diabetic patients with CAP and this in turn is associated with poor metabolic control. This contribution allows us to call attention to the importance of evaluating the presence of CAP and to emphasize not only the evaluation of LV diastolic function but also right ventricular diastolic function as an initial diabetes-related alteration independently of AHT, CAD, or valvular heart disease. Similarly, the findings of our study show the importance of achieving adequate metabolic control to avoid the appearance or mitigate the deleterious effects of CAP and ventricular dysfunction related to diabetic cardiomyopathy, either by establishing stricter glycemic control or by using specific treatments, taking into account Sodium-Glucose Cotransporter Type 2 (SGLT2) inhibitors and the broad benefits demonstrated by this pharmacological group in reducing the number of hospitalizations for heart failure in diabetic patients.
Acknowledgement
None.
Conflict of Interest
None.
References
- (2018) Introduction: Standards of Medical Care in Diabetes-2018. American Diabetes Association. Diabetes Care 41(Suppl. 1): S13-S27.
- Juan José Chillaróna, Juan Francisco Canoa B, Juan Pedro Botet B (2008) Type 1 diabetes mellitus and cardiovascular risk. Clin Invest Arterioscl 20(5): 210-217.
- Paschou SA, Papadopoulou Marketou N, Chrousos GP, Kanaka Gantenbein C (2018) On type 1 diabetes mellitus pathogenesis. Endocr Connect 7(1): R38-R46.
- (1984) World Health Organization. WHO Expert Committee on Cardiomyopathies. WHO Tech Rep Ser 697: 7-64.
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, AW Branwood, et al. (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30(6): 595-602.
- Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well controlled type 2 diabetes mellitus. Am J Cardiol 87(3): 320-323.
- Di Bonito P, Cuomo S, Moio N, Sibilio G, Sabatini D, et al. (1996) Diastolic dysfunction in patients with non-insulin-dependent diabetes mellitus of short duration. Diabetic Med 13(4):321-324.
- Beljic T, Miric M (1994) Improved metabolic control does not reverse left ventricular filling abnormalities in newly diagnosed non-insulin-dependent diabetic patients. Acta Diabetology 31(3): 147-150.
- Nicolino A, Longobardi G, Furgi G, Rossi M, Zoccolillo N, et al. (1995) Left ventricular diastolic filling in diabetes mellitus with and without hypertension. Am J Hypertens 8(4 Pt 1): 382-389.
- Boyer JK, Thanigaraj S, Schechtman KB, Perez JE (2004) Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 93(7): 870-875.
- Ignacio Gil Ortega, Juan Carlos Kaski (2006) Diabetic cardiomyopathy. Review Med Clin (Barc) 127(15): 584-594.
- Spallone V, Ziegler D, Freeman R, Luciano Bernardi, Simona Frontoni, et al. (2011) Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis and management. Diabetes/Metabolism Research and Reviews 27(7): 639-653.
- Cosson S, Kevorkian JP (2003) Left ventricular diastolic dysfunction: an early sign of diabetic cardiomyopathy? Diabetes Metab 29(5): 455-66.
- Monteagudo PT, Moises VA, Kohlmann O, Ribeiro AB, Lima VC, et al. (2000) Influence of autonomic neuropathy upon LV dysfunction in insulin-dependent diabetic patients. Clin Cardiol 23(5): 371-375.
- Porier P, Bogaty P, Garneau C, Marois L, Dumesnil JG (2001) Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 24(1): 5-10.
- Lazoglu AH, Glace B, Gleim GW, Coplan NL (1996) Exercise and heart rate variability. Am Heart J 131(4): 825-826.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS (2003) Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 42(5): 831-838.
- (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93(5): 1043-1065.
- Soedamah Muthu S, Fuller J, Mulnier H, Veena S Raleigh, Ross A Lawrenson, et al. (2006) High risk of cardiovascular disease in patients with type 1 diabetes in the U.K. Diabetes Care 29(4): 798-804.
- Irace L, Iarussi D, Guadagno I, Tedesco MA, Perna B, et al. (1996) Left ventricular performance and autonomic dysfunction in patients with long-term insulin-dependent diabetes mellitus. Acta Diabetologist 33(4): 269-273.
- Willenheimer RB, Erhardt LR, Nilsson H, Lilja B, Juul Möller S, et al. (1998) Parasympathetic neuropathy associated with left ventricular diastolic dysfunction in patients with insulin-dependent diabetes mellitus. Scand Cardiovasc J 32(1): 17-22.
- Monteagudo P, Valdir AM, Kohlmann O, Ribeiro A, Lima V, et al. (2000) Influence of Autonomic Neuropathy upon Left Ventricular Dysfunction in Insulin-Dependent Diabetic Patients. Clin. Cardiol 23(5): 371-375.
- Didangelos TP, Arsos GA, Karamitsos D, Athyros V, Karatzas N (2003) Left Ventricular Systolic and Diastolic Function in Normotensive Type 1 Diabetic Patients with or Without Autonomic Neuropathy. Diabetes Care 26(7): 1955-1960.
- American Diabetes Association (2020) Classification and diagnosis of diabetes: In Standards of Medical Care in Diabetes-2020. Diabetes Care 43 (Suppl. 1): S14-S31.
- (2011) World Health Organization. Hemoglobin concentrations for diagnosing anemia and assessing its severity. Geneva.
- Williams B, Mancia G, Spiering W, Enrico Agabiti Rosei, Michel Azizi, et al. (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39(33): 3021-3104.
- Lawrence G Rudski, Wyman W Lai, Jonathan Afilalo, Lanqi Hua, Mark D Handschumacher (2010) Guidelines for Echocardiographic Evaluation of the Right Chambers in the Adult: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiograph 23(7): 685-713.
- Roberto M Lang, Luigi P Badano, Victor Mor Avi, Jonathan Afilalo, Anderson Armstrong, et al. (2015) Recommendations for the Quantification of Cardiac Cavities by Echocardiography in Adults. J Am Soc Echocardiograph 28(1): 1-39.
- Roberto Flórez Gómez (2016) Journal of Echocardiography. Practice and other cardiac imaging techniques. Cardiac Imaging Unit. La Paz University Hospital. Madrid. Spain. RETIC 3: 63-65.
- Tanya Dutta, Wilbert S Aronow (2017) Echocardiographic evaluation of the right ventricle: Clinical Implications. Clinical Cardiology 40(8): 542-548.
- Ziegler D, Laux G, Dannehl K, M Spüler, H Mühlen, et al. (1992) Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabetic Medicine 9(2): 166-175.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS (1999) Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341(18): 1351-1357.
- American Diabetes Association (2020) Glycemic targets. Sec. 6. In Standards of Medical Care in Diabetes-2020. Diabetes Care 43(Suppl. 1): S66-S76.
- (2020) cardiovascular disease and risk management. Sec. 10. In Standards of Medical Care in Diabetes-2020. American Diabetes Association. Diabetes Care 43(Suppl. 1): S111-S134.
- (2017) World Health Organization. BMI classification.
- Okutucu S, Nadir U, Aytemir K, Ali Oto (2011) Heart rate recovery: A practical clinical indicator of abnormal cardiac autonomic function. Expert Review of Cardiovascular Therapy 9(11): 1417-1430.
- Turker Y, Aslantas Y, Aydin Y, Hilmi Demirin, Ali Kutlucan (2013) Heart rate variability and heart rate recovery in patients with type 1 diabetes mellitus. Acta Cardiologic 68(2): 145-150.
- Chaitman B (2003) Abnormal heart rate responses to exercise predict increased long-term mortality regardless of coronary disease extent: the question is why? Journal of the American College of Cardiology 42(5): 839-841.
- Morshedi Meibodi A, Larson M, Levy D, Christopher JO Donnell, Ramachandran S Vasan (2002) Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). The American Journal of Cardiology 90(8): 848-852.
- Seshadri N, Acharya N, Lauer M (2003) Association of diabetes mellitus with abnormal heart rate recovery in patients without known coronary artery disease. The American Journal of Cardiology 91(1): 108-111.
- Sacre J, Jellis C, Coombes J, TH Marwick (2012) Diagnostic accuracy of heart rate recovery after exercise in the assessment of diabetic cardiac autonomic neuropathy. Diabetic Medicine 29(9): 312-320.
- Devereux RB, Roman MJ, Paranicas M, MJ O Grady, ET Lee, et al. (2000) Impact of diabetes on cardiac structure and function: the Strong Heart Study. Circulation 101(19): 2271-2276.
- Kosmala W, Colonna P, Przewlocka Kosmala M, Mazurek W (2004) Right ventricular dysfunction in asymptomatic diabetic patients. Diabetes Care 27(11): 2736-2738.
- Karamitsos TD, Karvounis HI, Dalamanga EG, Christodoulos E Papadopoulos, Triantafyllos P Didangellos, et al. (2007) Early diastolic impairment of diabetic heart: the significance of right ventricle. Int J Cardiol 114(2): 218-223.
- Widya R L, Van Der Meer R W, Smit J W, Luuk J Rijzewijk, Michaela Diamant, et al. (2013) Right ventricular involvement in diabetic cardiomyopathy. Diabetes Care 36(2): 457-462.
- Kosmala W, Przewlocka Kosmala M, and Mazurek W (2007) Subclinical right ventricular dysfunction in diabetes mellitus-an ultrasonic strain/strain rate study. Diabet Med 24(6): 656-663.
- Khattab AA, Soliman MA (2015) Biventricular function and glycemic load in type 1 diabetic children: Doppler tissue-imaging study. Pediatric Cardiol 36(2): 423-431.
- Ghio S, Gavazzi A, Campana C, Inserra C, C Klersy, R Sebastiani, et al. (2001) Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 37(1): 183-188.
- Aziz EF, Kukin M, Javed F, Musat D, Amjad Nader, et al. (2010) Right ventricular dysfunction is a strong predictor of developing atrial fibrillation in acutely decompensated heart failure patients. ACAP-HF data analysis. J Card Fail 16(10): 827-834.
- Kahn K, Zola B, Juni JE, Vinik AI (1986) Radionuclide assessment of LV diastolic filling pressures in diabetes mellitus with and without cardiac autonomic neuropathy. J Am Coll Cardiol 7(6): 1303-1309.
- Candido R, Forbes JM, Thomas MC, Vicki Thallas, Rachael G Dean, et al. (2003) A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 92(7): 785-792.
- Bidasee KR, Zhang Y, Shao CH, Mu Wang, Kaushik P Patel, et al. (2004) Diabetes increases formation of advanced glycation end products on sarco-(endo) plasmic reticulum Ca2+-ATPase. Diabetes 53(2): 463-473.
- Tikellis C, Wookey PJ, Candido R, Andrikopsoulos S, Thomas MC, et al. (2004) Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes 53(4): 989-997.
- Santamore WP, Dell Italia LJ (1998) Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 40(4): 289-308.
- Cicala S, Galderisi M, Caso P, A Petrocelli, AD Errico, et al. (2002) Right ventricular diastolic dysfunction in arterial systemic hypertension: analysis by pulsed tissue Doppler. Eur J echocardiograph 3(2): 135-142.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.